The Non-Specificity of Cytopathic Effects: Implications for Virological Research and Public Health
By Matthew North - 13 February 2025
This study can be downloaded in pdf format by following this link.
The Non-Specificity of Cytopathic Effects: Implications for Virological Research and Public Health
Matthew North1
1USA, matthewnorth@finmail.com
Date: 13 February 2025
Keywords: Virology, Viral isolation, Cytopathic effects (CPE), Cell culture techniques, Uninoculated cultures, Control experiments, Methodological flaws
Abstract. This study critically examines the methodologies used for viral isolation, focusing on the reliance on cytopathic effects (CPE) as a primary indicator of viral presence. Standard virological techniques assume that CPE in cell cultures signifies viral replication; however, historical and contemporary control experiments challenge this assumption. Researchers such as Dr. Stefan Lanka and Jamie Andrews have demonstrated that CPE can occur in uninoculated cultures or due to non-viral factors, including antibiotic toxicity and nutrient deprivation. This calls into question the specificity of CPE-based isolation methods. Furthermore, a review of virological literature reveals methodological inconsistencies, particularly the lack of rigorous control experiments to differentiate viral effects from other cellular stress responses. These findings underscore the need for a reassessment of virological methodologies and a transition toward more robust and scientifically validated approaches. The implications extend beyond academic virology, influencing public health policies that rely on the accuracy of viral isolation techniques.
1 - Introduction
A previous study specifically evaluated the effect of varying concentrations of fetal bovine serum and antibiotics on CPE in control cultures [1]. The present study further examines the claim that CPE serves as an indicator of viral replication through a review of published literature.
The history of virus isolation is one marked by transformative milestones that have shaped modern virology. The journey began in 1892 with Dmitri Ivanovsky, whose experiments with the sap of infected tobacco plants revealed the existence of "filterable agents" [2]. Using a Chamberland filter, Ivanovsky demonstrated that these agents could pass through bacterial filters and remain infectious, challenging the prevailing understanding of pathogens.
This discovery was later refined by Martinus Beijerinck, who found that, unlike bacteria, a virus failed to grow in artificial media but remained infectious when introduced to healthy plants. Beijerinck observed that the agent could only replicate within living plant cells, relying on the host for reproduction [3]. He termed it a "contagium vivum fluidum" (living contagious fluid), emphasizing that it behaved differently from bacteria, reinforcing the idea that viruses were obligate intracellular parasites that required host cells to multiply.
The early 20th century saw the development of additional methods to study viruses, including advancements in filtration, gradient centrifugation, and the inoculation of host organisms [4]. However, the lack of direct visualization hindered efforts to isolate and characterize viruses. This limitation was addressed with the invention of the electron microscope in the 1930s, which allowed researchers to observe viral particles for the first time. Images of bacteriophages and animal viruses illuminated their structure and morphology providing what is claimed to be tangible evidence.
In 1954, John Enders and his colleagues ‘revolutionized’ virology by developing techniques to grow poliovirus in non-neural human and monkey tissue cultures. This breakthrough eliminated the need for live animal hosts, allowing for more ethical, efficient, and reproducible studies. Their work not only facilitated vaccine development but also established cell culture as the gold standard for virus isolation. This method paved the way for isolating and studying a wide range of viruses, significantly advancing virology. This isolation method has been built upon since the time of Enders, but it remains, in principle, much the same.
Cell cultures allow researchers to observe the CPE of viruses, study virus-host interactions. They are essential for detecting viable viral particles and assessing the infectivity of clinical specimens. Overall, cell culture remains integral to virology, providing valuable insights into viral behavior and facilitating the development of antiviral therapies and vaccines [5, 6]. However, despite the widespread use of CPE-based methods, significant methodological concerns remain. Many virological studies assume that CPE in cell cultures are direct evidence of viral presence and pathogenicity, yet historical and contemporary control experiments have demonstrated that CPE can occur in uninoculated cultures or due to factors unrelated to viral infection. The reliance on these indirect indicators raises questions about the specificity and reliability of viral isolation techniques. Additionally, studies by researchers such as Dr. Stefan Lanka and Jamie Andrews have highlighted the need for rigorous control experiments to account for potential non-viral causes of cellular changes.
This paper critically evaluates the methodologies used for viral isolation, particularly the reliance on CPE as a proxy for viral identification. By analyzing historical studies, control experiments, and the broader implications of methodological weaknesses, we aim to reassess the foundations of virology and promote a more rigorous scientific approach. The findings presented here have significant implications for virological research, public health strategies, and the interpretation of viral causation in disease.
2. Methodology
This literature review was conducted to analyze the methodologies used in viral isolation, focusing on their reliability, limitations, and implications in virology. A systematic search was performed across academic databases, including PubMed, Google Scholar, ScienceDirect, and NCBI, using keywords such as “viral isolation techniques,” “cytopathic effect (CPE) limitations,” and “control experiments in virology.” Peer-reviewed journal articles, review papers, and primary research studies published in English were prioritized. Studies discussing virus isolation methods, control experiments, and critiques of virological methodologies were included, while non-methodological papers and studies lacking transparency were excluded unless they provided unique insights.
3 – Virus Isolation: Importance and Techniques
Virus isolation is fundamental to virology for several reasons:
Identification and Characterization: Isolating viruses allows scientists to identify and characterize new viruses, understanding their structure, genetic makeup, and pathogenic mechanisms.
Study of Virus-Host Interactions: By isolating viruses, researchers can investigate how viruses infect cell cultures, replicate, and cause disease, which is crucial for understanding viral pathogenesis.
Vaccine Development: Virus isolation is essential for developing vaccines, as it enables the creation of attenuated or inactivated viruses for immunization.
Antiviral Drug Testing: Isolated viruses are used to test the efficacy of antiviral drugs, helping to develop treatments that inhibit viral replication.
Epidemiological Studies: Isolating viruses from clinical samples helps track the spread of viral diseases and understand their epidemiology, which is vital for public health responses.
Diagnostic Tools: Virus isolation is used to develop and validate diagnostic tests, ensuring they accurately detect specific viruses in clinical samples.
One of the most widely used methods for virus isolation is the cell culture technique, where the presence of a virus is indicated by CPE. The process involves the following steps:
Cell Culture Preparation: Cultivate a monolayer of susceptible cells in a culture medium.
Inoculation: Introduce the prepared sample containing the virus into the cell culture.
Incubation: Allow the virus to infect the cells and replicate under controlled conditions.
Observation of CPE: Monitor the cell culture for signs of CPE, which include morphological changes such as cell rounding, detachment, syncytia formation, and cell death. These effects indicate viral replication and infection.
Confirmation: Confirm the presence of the virus through additional tests, such as immunofluorescence, PCR, or electron microscopy [7, 8, 9, 10, 11, 12].
Figure 1: Virus isolation process – A basic breakdown
CPE is a critical indicator in virus isolation, as it provides visual evidence of viral activity within cell cultures. This method has been instrumental in identifying and studying numerous viruses, contributing significantly to our understanding of viral diseases and the development of treatments and preventive measures.
4 - Control Experiments in Virology
4.1 - Historical Control Experiments
The field of virology has long relied on the observation of CPE in cell cultures as evidence of viral presence and pathogenicity. However, numerous historical control experiments have demonstrated that CPE can occur in uninoculated cultures, raising significant questions about the validity of these methods. This section reviews control experiments that demonstrated CPE in uninoculated cultures and discusses their implications for virology. The studies that were reviewed include the following:
J. F. Enders and T. C. Peebles 1954 - Under other agents isolated during the study. "A second agent was obtained from an uninoculated culture of monkey kidney cells. The cytopathic changes it induced in the unstained preparations could not be distinguished with confidence from the viruses isolated from measles." And “While there is no ground for concluding that the factors in vivo (in the body) are the same as those which underlie the formation of giant cells and the nuclear disturbances in vitro (outside a living organism), the appearance of these phenomena in cultured cells is consistent with the properties that a priori might be associated with the virus of measles.”
Rustigian et al, 1955 - “In our attempts, in 1953, to adapt mouse-adapted Hawaii dengue virus (2) to roller tube cultures of rhesus monkey kidney, an unidentified agent was encountered which induced cytopathogenic changes in cultures of monkey kidney and cancer HeLa epithelial cells.”
Cohen et al, 1955 - “Controls consisted of uninoculated cultures and of cultures passaged serially with fluids from uninoculated tubes.” “Enders and Peebles (1) and Rustigian et al. (10) encountered latent virus-like agents that induce marked vacuolization and syncytial masses in monkey kidney tissue cultures. The cellular degeneration characteristic of these “monkey-kidney agents” frequently appeared in our cultures, both in those inoculated with specimens from measles patients and in controls; hence cytologic criteria for recognition of measles agents were difficult to apply.”
G. Henle et al, 1955 - “The use of monkey kidney cells for virus isolations presents certain problems. In the above studies, 11 batches of kidney cells were employed. In cultures of one of these, large “giant cells” appeared in control tubes after 11 days of incubation similar to those produced by the “foamy agent” (3,4). Prior to the 11th day, tubes inoculated with saliva from a mumps patient had shown lesions which were attributed to the presence of mumps virus. Since the cytopathogenic effects induced by these 2 agents thus far proved to be indistinguishable…”
S. Hotta et al, 1956 - “A cytopathogenic agent recovered from an apparently normal kidney tissue culture. A cytopathogenic agent was originally encountered in a control uninoculated culture tube of rhesus kidney tissue 7 days after the beginning of incubation.”
Federal Proceding, 1956 - Some people might defend John Enders by claiming he could differentiate the cytopathic effects in the cultures because “internuclear changes typical of the measles agents” was not observed in the uninoculated culture. However, such intranuclear changes do occur in uninoculated cultures as well. At the Federal Proceeding in 1956, Jonas Salk reported the findings of Gisela Ruckle, a virologist who discovered intranuclear inclusions identical to measles in uninoculated cultures. The uninoculated cultures were also immunologically indistinguishable from human measles virus. The proceeding concludes the following: “During these studies two different types of transmissible agents have been found in uninoculated monkey kidney tissue cultures. One resembles the so-called ‘foamy virus’ and the other produces intranuclear inclusions that are indistinguishable from that produced by the agent obtained from human measles. Each of these agents has been encountered on six different occasions and under circumstances where the possibility of contamination with human measles virus could be excluded … Cross neutralization tests reveal that the ‘foamy virus’ is different from the monkey intranuclear inclusion agent and that the latter is immunologically indistinguishable from human measles virus.”
T. H. Weller, 1956 – “Gey (Gey et al., 1954) has emphasized that so-called “non-specific” degeneration in a tissue culture may signify the presence of a viral contaminant and has expressed the view that stable cell strains maintained in homologous media are in particular jeopardy.”
L. V. Brown, 1957 - “In recent years, with the increased use of tissue cultures prepared from monkey kidney cells, a new group of viruses has come into recognition. The presence of these agents is made known by the cytopathic effect which they produce in uninoculated or control cultures. The combined observations of workers in several laboratories indicate that these agents, as yet unclassified, are unaccountably present in the kidney tissues (or blood elements) of the apparently normal, healthy monkeys from which the cultures are derived.” and “A cellular degeneration of peculiar type, featuring the formation of “blisters” or “foamy” patches and of multinucleated giant cells, has been seen to occur spontaneously in tissue cultures prepared from the kidneys of apparently normal, healthy rhesus (Macaca mulatto) or cynomologus (Macaca irus mindanensis) monkeys”
G Ruckle, 1958 - “Early in the study of measles virus in monkey kidney tissue, a second agent was encountered in uninoculated monkey kidney cultures, which was, in its cytopathic capacity, indistinguishable from measles virus and provisionally referred to as monkey-intra-nuclearinclusion-agent (MINIA) … This paper describes studies to investigate the tissue culture behavior of MINIA and foamy-agent, their immunologic relationship to each other and to measles virus.” She observed cytopathic effects identical with those attributed to measles in control cultures, as well as in cultures inoculated with material other than from measles patients. She stated that the changes seen in the uninoculated cultures had previously only been associated with measles virus. “The examination of the stained preparations of one batch prepared on June 23, 1955, revealed the presence of cytopathic changes identical with those induced by measles virus in the control, as well as in the cultures inoculated with clinical material other than from measles patients … A greater number of uninoculated cultures derived from cell batches prepared on June 29, 30 and July 8, were examined and also showed changes, in a number of cultures, which had been associated until this time only with measles virus.” She concluded that the alleged “agent” called MINIA was indistinguishable from measles virus. “MINIA produces identical cytopathic changes in monkey kidney cultures as does measles virus … The immunological properties of 8 MINIA strains investigated were indistinguishable and identical with those of human measles virus.”
G Ruckle, 1958 - “The recovery of MINIA and foamy-agent from spontaneously degenerating cultures of monkey kidney tissue has been previously reported. MINIA has been shown to be immunologically indistinguishable from human measles virus and to produce the same unique cytopathic changes in human kidney, monkey kidney and human amniotic membrane cell cultures … MINIA and foamy-agent are agents which have been recovered from spontaneously degenerating monkey kidney cultures. Tissue-culture behavior and immunological properties of both agents were distinct and MINIA was identified as being indistinguishable from measles virus.”
G. D. Hsiung, et al, 1958 – “Foamy agents have been confused with measles virus because of the characteristic cytopathic changes in rhesus monkey kidney cultures in fluid medium.”
Bech and von Magnus, 1959 - “Increased use of the technique of cell cultivation for isolation, maintenance and study of viruses has resulted in the discovery of many hitherto unknown cytopathogenic agents”
F Rapp et al, 1959 - Most noteworthy is “Monkey kidney cells, however, are unsuitable for the investigations of the type reported here; Peebles et al. and Ruckle showed that monkeys, and cell cultures derived from them, are often infected with an agent serologically indistinguishable from human measles virus, which causes cytopathic changes in monkey kidney cell cultures almost identical with those caused by human measles virus.”
F.L. Black et al, 1959 - “Of the two tissue culture systems first used successfully by Enders and Peebles (1954), rhesus or cynomolgus monkey kidney is the easier for most laboratories to obtain. Unfortunately, early in the course of this work it was found that agents which induced cytopathic effects superficially resembling that of measles virus occurred in uninoculated cultures. The original observations have since been confirmed and extended by Rustigian et al. (1955), Ruckle (1958), and Brown (1957).” The authors concluded that the cell culture method cannot demonstrate the existence of a measles virus because the same effects are seen in uninoculated cultures. “In addition to the foamy viruses, other agents that are identical to measles virus in terms of their serological relationships, cytopathological effects and range of tissue culture susceptibility have been found in uninoculated cultures (Ruckle, 1956; Brown, 1957). In view of these complications, cultures of monkey kidneys cannot be considered a suitable tool for the isolation or propagation of measles virus. Even if, by careful serological and cytological tests, one identifies an agent grown in monkey kidney as measles virus, there can be no certainty that it did not derive from the cultures themselves.”
P. B. Johnston, 1961 - “During the last 6 years there have been many reports of isolation of simian “foamy” virus from “uninoculated” kidney cell cultures of rhesus and cynomolgus monkeys (Enders and Peebles 1954; Rustigian et al, 1955; Henle and Deinhardt, 1955; Hotta and Evans, 1956; Brown, 1957; Falke, 1958; Ruckle, 1958a; Endo et al, 1959) and from African monkeys and baboons (Hsiung et al, 1958; Lepine and Paccaud, 1957). Similar virus was found by Weller (1956) in “spontaneously” degenerating (uninoculated) cultures of monkey testis.”
G Ruckle, 1962 - “The preceding studies show that the 2 agents, measles virus and MINIA, behave identically with respect to their biological, chemical, antigenic, and epidemiological properties and can be considered as homogeneous agents.” Without any evidence, the authors claim that the uninoculated culture must have been “contaminated” with a measles virus.
M. D. Eaton et al, 1962 - “Experiments with yeast extract. Wittler, Cary, and Lindberg (1956) reported that yeast extract speeds up the growth of PPLO in tissue cultures and increases the cytopathic effect. When yeast extract (Difco) at a concentration of 0.5% was present in our cultures at the time of inoculation, both the control and the inoculated cultures showed degenerative changes within 1 week, probably because of toxicity of the extract for the cells.”
E. C. Dick, 1963 - “During prolonged incubation (2 weeks or more) of uninoculated chimpanzee kidney tissue cultures, the cells frequently exhibited changes similar to changes caused by the growth of viruses.”
H. Malherbe and R. Harwin, 1963 - “The introduction of tissue culture methods for the study of poliovirus by Enders, Weller and Robbins in 1949, and the subsequent development of the monolayer cell technique by Youngner, led to the discovery of many viruses. A number of these have been found in uninoculated tissue cultures, and it is helpful to have some knowledge of the viruses likely to be encountered in any particular animal, to avoid ascribing autochthonous agents to materials inoculated into cultures, and to reduce the danger of infection to persons handling cultures or animals.” and “The virus has been recovered from mouth swabs and from uninoculated kidney cultures. It is present in small amounts in the kidneys of many monkeys, and if cultures are held for prolonged periods a higher isolation rate results.” and “Serologically identical strains of this virus have been isolated from uninoculated monkey kidney cultures in the summer months of two years, and the properties of the virus suggest that it is an enterovirus. Features common to other enterovirus infections are noted in the cells (Fig. 4): a large cytoplasmic paranuclear mass displaces the nucleus, which becomes scrolled or crushed. Small eosinophilic bodies may be seen in the nucleus in addition to nucleolar remnants.”
G. D. Hsiung, 1968 - “To our surprise, measles virus intranuclear and intracytoplasmic eosinophilic inclusions occurred in both inoculated and uninoculated control HEK cultures. Thus, the adenovirus stock derived from the commercially made HEK cultures was inadvertently contaminated with a measles virus.”
G. D. Hsiung, 1968 - “Much to our surprise, an unusually high percentage of cultures that were considered “normal” showed virus infection.”
A. B. Nesbern, 1968 - “An agent which possesses the physical, chemical, cytopathic, histological, and electron microscopic attributes of a herpes group virus was isolated from an uninoculated batch of primary rabbit kidney cell cultures.” and “ Negatively stained preparations contained particles of the shape, size, and configuration described for other herpes group viruses (15, 23; Fig. 5 and 6).”
S. Makino et al, 1970 - “We found that goat kidney cells were also highly susceptible to measles virus, but uninoculated cultures also developed cytopathic effects frequently.”
R. F. Smith et al, 1970 - “Puppies serving as donors of kidney tissues for the cell cultures were derived from a closed colony of apparently healthy beagle dogs. The primary canine kidney cells were placed on maintenance medium consisting of Eagle’s minimal essential medium plus 2% fetal calf serum on the day after receipt of the monolayer cultures. Foci of CPE consisting of rounded cells and plaques (Fig. IA) appeared at 14 days after initiation of the cultures. The CPE progressed to involve 50 to 75% of the cell sheet by the end of the third week of cultivation.”
D. A. Tyrrell et al, 1983 - “We have found that CSFs from patients with certain psychiatric syndromes, including schizophrenia, produce a cytopathic effect (CPE) when inoculated into stationary tissue cultures. This CPE resembles that produced by certain viruses, but no cytopathic agent has yet been established in tissue culture.”
H. H. Yoshimura et al, 1984 - “Intestinal tissue filtrates induce cytopathic effects in inoculated cell cultures, but the effect we observed is non-specific … Our results suggest that the observed cytopathic effect was caused by a non-replicating cytotoxic factor.”
Carl J. O’Hara et al, 1988 - The study demonstrated "HIV" particles in 18 out of 20 (90% of) AIDS-related lymph node enlargements but also in 13 out of 15 (88% of) non-AIDS-related enlargements. Which means that particles claimed to be HIV virions are non-specific since identical particles can be found in the majority of patients with enlarged lymph nodes not attributed to AIDS, and at no risk for developing AIDS.
P Gluschankof et al, 1997 - Who found particles identical to those claimed to be the HIV virus in uninoculated cultures. Refer to the electron micrographs.
Julian W. Bess Jr., 1997 - Who found particles identical to those claimed to be the HIV virus in uninoculated cultures. Refer to electron micrographs as well as the proteomics.
C.A. Cassol, 2020 - “We have observed morphologically indistinguishable inclusions within podocytes and tubular epithelial cells both in patients negative for coronavirus disease 2019 (COVID-19) as well as in renal biopsies from the pre-COVID-19 era”.
L Caly, et al, 2020 - “Following several failures to recover virions with the characteristic fringe of surface spike proteins, it was found that adding trypsin to the cell culture medium immediately improved virion morphology.”
4.2 - Contemporary Control Experiments
4.2.1 - Control Experiments Carried Out by Stefan Lanka
In order to demonstrate the shortcomings of the isolation method Dr. Stefan Lanka carried out control experiments [13]. His phase 1 control experiment focuses on the CPE observed in cell cultures, which virologists traditionally attribute to the presence of pathogenic viruses. The experiment aimed to demonstrate that CPE can occur without the presence of infectious material, thereby questioning the validity of using CPE as proof of viral isolation. Some of the findings are illustrated in the below figures.
Representative micrographs of the 4 experimental groups of epithelial cells at passage 4 and 6 are illustrated. From left to right:
Healthy control cells with 1 x triple antibiotics in control medium (CM) or DMEM/GlutaMAX with 10% FCS; stressed cells with 3x Triple antibiotics and 1% FCS in DMEM.
The cells in the right panel were treated with total yeast RNA (yRNA) for 1 h before changing the media.
(A), (B) Cells in expansion for the purpose of RNA isolation. Note that the CPE becomes more prominent over the three passages. (B) Top row: cells before medium change. (C), (D) Confluent cells for visualization of CPE; (C) Top row: confluent cells before medium change. (D) Cell cultures from 3 biological replicates stained with crystal violet at the time of harvest. Note that the cells in the two left panels form a continuous cell sheet while the cells in the two right panels have high plaque counts (arrows) compatible with significant CPE increasing from day 1 to day 5. Cultures treated with yeast RNA show a significantly higher number of larger plaques.
Key Findings
Non-Specificity of Cytopathic Effect (CPE):
The experiment revealed that CPE, characterized by cell rounding, detachment, and death, can be induced in cell cultures without the presence of any infectious material. This finding challenges the traditional interpretation that CPE is specific to viral infection.
Replication of Historical Observations:
The experiment confirmed earlier observations by John F. Enders and others that CPE could occur due to factors unrelated to viruses, such as cell starvation and poisoning. This replication of historical findings underscores the need for rigorous control experiments in virology.
Impact on Experimental Conditions:
Various non-viral and non-infectious substances, including high concentrations of antibiotics (penicillin/streptomycin), lipopolysaccharides, and material from throat swabs, were added to the cell cultures. These conditions led to changes in cell morphology that were microscopically identical to those attributed to viral infection.
The addition of high concentrations of antibiotics or cultivation under nutrient-deficient conditions (1% fetal calf serum) resulted in significant CPE, indicating that these effects were not virus-specific.
Confirmation of Non-Virus Specificity:
The experiment demonstrated that the morphological changes in cell cultures, such as syncytia formation (cell fusion and subsequent death), were not specific to measles infection or any other viral infection. This finding was consistent with earlier studies by Bech and von Magnus, which showed that similar CPE could be observed in uninoculated cultures of monkey kidney tissue.
Role of Antibiotics and Nutrient Deprivation:
The use of antibiotics, particularly streptomycin, was found to damage mitochondria within the cells, leading to cell death. This effect was previously known but is often overlooked in virological studies.
Nutrient deprivation, achieved by reducing the concentration of fetal calf serum, also contributed to the observed CPE. This starvation effect was intended to make cells more susceptible to viral infection but was shown to cause cell death independently.
Implications for Virology:
The findings suggest that the traditional method of using CPE as proof of viral isolation is flawed. The observed CPE can be caused by various factors unrelated to viral infection, such as experimental conditions and the presence of antibiotics.
This challenges the validity of many virological studies that rely on CPE as evidence of viral presence and calls for a reevaluation of existing methodologies.
Lack of Control Experiments:
The experiment highlighted that many virologists do not perform the necessary control experiments to rule out non-viral causes of CPE. This lack of rigorous controls has led to misinterpretations and false conclusions about the presence and pathogenicity of viruses.
Broader Implications:
The findings have significant implications for the field of virology, suggesting that many claims about virus isolation and pathogenicity may be based on flawed methodologies. This calls for more rigorous scientific practices and a reassessment of existing virological research.
Dr. Stefan Lanka's phase 1 control experiment provides compelling evidence that the CPE observed in cell cultures is not specific to viral infection and can be induced by various non-viral factors. These findings challenge the traditional methods used in virology for virus isolation and highlight the need for more rigorous control experiments to ensure the validity of scientific conclusions. The experiment underscores the importance of critical evaluation and validation in scientific research, with profound implications for the field of virology.
4.2.2 - Control Experiments Carried Out by Jamie Andrews
This section summarizes a series of control experiments designed to critically evaluate the validity of virus isolation methodologies. The experiments were carried out by Jamie Andrews and aimed to demonstrate that the observed CPE in cell cultures, traditionally attributed to viral presence, can occur in the absence of any viral inoculum [1]. The experiments were also designed to improve on the work of Dr Lanka (refer to Section 4.2.1) and address critiques that claimed that Dr Lanka used fragile epithelial cells that would more readily induce CPE. HEK293T cells were used instead which are believed to be generally more resilient due to their transformed nature, rapid growth, and adaptability to culture conditions.
Nine experiments were conducted to observe CPE in uninfected HEK293T cell cultures under varying conditions of fetal bovine serum (FBS) and antibiotic concentrations. The experiments included both negative controls, maintained in DMEM with 10% FBS and 1x Penicillin/Streptomycin (P/S), and test cultures with reduced FBS and varying P/S levels. The images captured at different magnifications (10x and 20x) revealed significant CPE in the test cultures, including plaque formation, ballooning, rounding, floating, lifting, and syncytia. These effects were observed as early as 48 hours into incubation and increased over time, with the extent of CPE ranging from approximately 10% to 40% cell death.
Experiment 9 of the study is illustrated in Figure 2 and Figure 3 with notes as follows:
Figure 2 (negative control) shows cells normally growing, near confluent. 20x magnification.
Figure 3 (test) show:
A: Cells dying, many floating. Apoptosis, CPE and syncytia observed.
B: Many cells dying/lifting/floating. CPE/apoptosis/syncytia observed.
C: Cells dying/lifting/floating. Cells other than HEK observed, likely from sputum. CPE/apoptosis/syncytia observed.
A, B and C 20x magnification
Negative Control
Figure 2: HEK 293T Day 4 DMEM 10%FBS, 1x P/S.
Figure 3: A: 1X Pen/Strep, HEK 293T Day 4 DMEM 2%FBS, B: 1X Pen/Strep/2X Amphotericin, HEK 293T Day 4 DMEM 2%FBS, C: 1X Pen/Strep/2X Amphotericin, HEK 293T Day 4 DMEM 2%FBS + 350ul Sputum.
The study further details the Transmission Electron Microscopy (TEM) analysis conducted by an independent Contract Research Organization (CRO) on one of the control cultures. The TEM images were inspected to identify viral-like particles based on their size, shape, and protein inclusions, as defined by the Centers for Disease Control and Prevention (CDC) for viruses such as SARS-CoV-2, measles, and HIV. The analysis revealed particles in the control cultures that matched the characteristics of these known viruses, particularly in terms of size and overall shape. For instance, particles resembling SARS-CoV-2 Omicron variant, measles virus, and HIV were identified, challenging the assumption that these particles are exclusive indicators of viral presence. Figure 4 to Figure 9 are presented in the study.
Figure 4: Particle of exactly the same size, shape and protein inclusions as the CDC image below of SARS-CoV-2, Omicron BA.2 (refer to Figure 5).
Figure 5: (Left) Low magnification electron micrograph of a monkey kidney cell (Vero E6) after infection with the SARS-CoV-2 Omicron variant showing cell damage with swollen vesicles containing small black viral particles. (Right) High magnification electron micrograph of an infected Vero E6 cell showing aggregates of viral particles with corona shaped spikes on their surface (red box). Photo credit: Professor John Nicholls, Clinical Professor of Department of Pathology; and Professor Malik Peiris, Tam Wah-Ching Professor in Medical Science and Chair Professor of Virology, School of Public Health, HKUMed; and Electron Microscope Unit, HKU.
Figure 6: A particle of exactly the same size, shape and protein inclusions as the CDC image below of Measles Virus (refer to Figure 7).
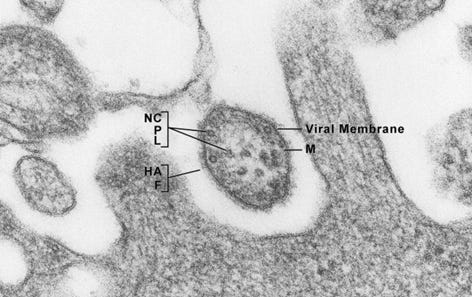
Figure 7: This transmission electron microscopic (TEM) image revealed the ultrastructural appearance of a measles virus particle, or virion, a Paramyxoviridae family member, and a member of the genus, Morbillivirus. The viral particle is 100-200 nm in diameter, with a core of single-stranded RNA, and is closely related to the rinderpest, and canine distemper viruses. Two membrane envelope proteins are important in pathogenesis. They are the F (fusion) protein, which is responsible for fusion of virus and host cell membranes, viral penetration, and hemolysis, and the HA (hemagglutinin) protein, which is responsible for adsorption of virus to cells.
Figure 8: A particle of exactly the same size, shape and protein inclusions as the CDC image below of HIV Virus (refer to Figure 9).
Figure 9: This highly magnified transmission electron micrographic (TEM) image revealed the presence of mature forms of the human immunodeficiency virus (HIV) in a tissue sample under investigation. HIV is a retrovirus, identified in 1983 as the etiologic agent for the acquired immunodeficiency syndrome (AIDS). AIDS is characterized by changes in the population of T-cell lymphocytes that play a key role in the immune defense system. In an infected individual, the virus causes a depletion of subpopulation of T-cells, called T-helper cells, which leaves these patients susceptible to opportunistic infections, as well as certain malignancies.
Key Findings
Non-Specificity of Cytopathic Effect (CPE)
The study demonstrated that significant CPE, such as plaque formation, ballooning, rounding, floating, lifting, and syncytia, can occur in uninfected HEK293T cell cultures. These effects, traditionally attributed to viral infections, were replicated by varying the culture environment, challenging the assumption that CPE is specific to viral presence.
Replication of Historical Observations
The findings align with historical observations that CPE can occur in uninoculated cultures. This replication supports the notion that morphological changes in cell cultures are not exclusive to viral infections and can be induced by other factors.
Impact on Experimental Conditions
The study found that reduced FBS environments and varying antibiotic concentrations significantly influenced the extent of CPE in uninfected cultures. This highlights the critical role of the culture environment in inducing morphological changes, traditionally attributed to viral infections.
Confirmation of Non-Virus Specificity
TEM analysis revealed the presence of viral-like particles in control cultures, resembling known viruses such as SARS-CoV-2, measles, and HIV. This finding challenges the specificity of these particles as indicators of viral presence, suggesting that they may not be exclusive to viral infections.
Role of Antibiotics and Nutrient Deprivation
The study demonstrated that nutrient deprivation (reduced FBS) and varying antibiotic concentrations (particularly Penicillin/Streptomycin) play a critical role in inducing CPE. These conditions, commonly used in viral isolation protocols, were shown to significantly impact cell viability and morphology.
Implications for Virology
The findings challenge the traditional reliance on CPE as definitive indicators of viral presence. The study calls for a reassessment of current viral isolation protocols, emphasizing the need for alternative methods that do not rely solely on CPE as an indicator.
Lack of Control Experiments
The study highlighted the lack of rigorous control experiments in virological research. The findings underscore the importance of including appropriate controls to accurately interpret the results and avoid misattributing CPE to viral presence.
Broader Implications
The broader implications of the study suggest that many claims about virus isolation and pathogenicity may be based on flawed methodologies. The findings call for more rigorous scientific practices and a critical reassessment of virological paradigms to ensure the accuracy and reliability of virological research. This has significant implications for public health policies and interventions, which often rely on the assumption of transmissible viral pathogens.
Jamie’s control experiments demonstrated that significant CPE, such as plaque formation, ballooning, rounding, floating, lifting, and syncytia, typically attributed to viral infections, could be replicated in uninfected cultures by manipulating the culture environment. TEM analysis revealed the presence of viral-like particles in control cultures, resembling known viruses such as SARS-CoV-2, measles, and HIV. These findings challenge the assumption that CPE and viral-like particles are exclusive indicators of viral presence, suggesting that the culture environment itself plays a significant role in their manifestation. The study calls for a critical reassessment of current viral isolation methods and the interpretation of CPE in cell cultures, emphasizing the need for alternative methods that do not rely solely on CPE as an indicator.
5. Discussion
The findings presented in this paper raise significant questions about the reliability of current methodologies used in virology, particularly the reliance on cytopathic effects (CPE) as a primary indicator of viral presence. By systematically reviewing historical and contemporary control experiments, we have demonstrated that CPE can occur in the absence of viral infection, challenging the foundational assumptions of many virological studies. This discussion explores the implications of these findings, the limitations of current methods, and the need for a more rigorous scientific approach in virology.
5.1 - Non-Specificity of Cytopathic Effects (CPE)
One of the most critical findings of this paper is the non-specificity of CPE as an indicator of viral infection. Historical studies, such as those by Enders and Peebles (1954) and Ruckle (1958), have shown that CPE can occur in uninoculated cell cultures, often due to factors unrelated to viral activity. These findings were replicated in contemporary control experiments by Dr. Stefan Lanka and Jamie Andrews, who demonstrated that CPE could be induced by non-viral factors such as nutrient deprivation, high concentrations of antibiotics, and other stressors in the cell culture environment.
The observation that CPE can be caused by non-viral factors undermines the traditional interpretation that cellular changes are definitive evidence of viral replication. This non-specificity calls into question the validity of many virological studies that rely solely on CPE to confirm the presence of a virus. If CPE can be induced by factors such as cell starvation or antibiotic toxicity, then the assumption that CPE is a direct result of viral infection becomes untenable without rigorous control experiments.
5.2 - Implications for Virology
The implications of these findings are profound for the field of virology. If CPE is not a reliable indicator of viral presence, then many of the foundational studies that have shaped our understanding of viral diseases may need to be re-evaluated. This is particularly concerning for studies that have led to the development of vaccines, antiviral drugs, and diagnostic tests, all of which rely on the accurate isolation and identification of viruses.
For example, the development of the polio vaccine by John Enders and colleagues was based on the observation of CPE in cell cultures. The methodological concerns raised in this paper suggest that the interpretation of CPE as evidence of viral infection may have been overly simplistic. Similar concerns apply to other viruses, such as SARS-CoV-2, measles and HIV, where CPE has been used as a key indicator of viral replication.
The broader implication is that virology as a discipline must adopt more rigorous scientific practices, particularly in the design and interpretation of control experiments. Without proper controls, it is impossible to rule out non-viral causes of CPE, leading to potential misattributions of cellular changes to viral activity. This has significant consequences for public health policies, which often rely on the assumption that viruses are the primary causative agents of disease.
5.3 - Limitations of Current Methods
The reliance on CPE as a proxy for viral presence is a significant limitation of current virological methods. While cell culture techniques have been instrumental in advancing our understanding of viruses, they are not without flaws. The findings of this paper highlight several key limitations:
Lack of Specificity: CPE can be induced by a wide range of non-viral factors, including antibiotics, nutrient deprivation, and other stressors in the cell culture environment. This lack of specificity makes it difficult to conclusively attribute cellular changes to viral infection.
Inadequate Control Experiments: Many virological studies do not include rigorous control experiments to rule out non-viral causes of CPE. This oversight can lead to false conclusions about the presence and pathogenicity of viruses.
Misinterpretation of Viral-Like Particles: Transmission Electron Microscopy (TEM) studies have revealed that viral-like particles can be present in control cultures, even in the absence of viral infection. This challenges the assumption that these particles are exclusive indicators of viral presence and bring into question the only tangible evidence for viruses.
Overreliance on Indirect Evidence: The use of CPE as an indirect indicator of viral infection is problematic, as it does not provide direct evidence of viral replication. It also questions the validity of downstream methods, such as genetic sequencing, which would be difficult to undertake without being able to obtain a pure virus sample.
5.4 - Future Directions
To address these limitations, the field of virology must adopt more rigorous and transparent methodologies. Several areas for future research and methodological improvement are suggested:
Improved Control Experiments: Virological studies must include rigorous control experiments to rule out non-viral causes of CPE. This includes testing the effects of antibiotics, nutrient deprivation, and other stressors in the cell culture environment.
Replication of Historical Studies: Many foundational studies in virology should be replicated using modern techniques and rigorous controls to confirm their findings. This is particularly important for studies that have led to the development of vaccines and antiviral drugs.
Transparency and Open Science: Virological research should prioritize transparency and open science practices, including the publication of raw data, experimental protocols, and control experiments. This will allow for independent verification of results and reduce the risk of misinterpretation.
5.5 - Broader Implications for Public Health
The findings of this paper have significant implications for public health policies that rely on the accurate identification of viral pathogens. If current methods for virus isolation are flawed, then the assumptions underlying many public health interventions may need to be re-evaluated. For example, the development of vaccines and antiviral drugs depends on the accurate identification of viral targets. If these targets are misidentified due to methodological flaws, the effectiveness of these interventions may be compromised.
Furthermore, the interpretation of disease causation in virology has far-reaching consequences for public health. If viruses are not the primary causative agents of certain diseases, then alternative explanations, such as environmental or metabolic factors, must be explored. This could lead to a paradigm shift in how we understand and treat diseases.
6 - Conclusion
Notably, a study published on February 5, 2025, identifies significant empirical gaps and methodological limitations in demonstrating natural person-to-person transmission of viruses [14]. This lack of consistent evidence challenges the foundational assumption of viral transmissibility.
This study further demonstrates that reliance on CPE, a foundational method in virology, presents methodological limitations and inconsistencies. The non-specificity of CPE, combined with the lack of rigorous control experiments, calls into question the validity of many virological studies. To address these concerns, the field of virology must adopt more rigorous scientific practices, including the development of more specific indicators of viral presence, improved control experiments, and greater transparency in research.
By critically evaluating the methodologies used in virology, this paper advocates for a more robust and scientifically rigorous approach to the study of viruses. The findings presented have profound implications for virology as a discipline and for public health policies that depend on the accurate identification of viral pathogens. It is hoped that this study will inspire further research and debate, contributing to a more accurate and reliable understanding of diseases.
7 - Acknowledgements
The author would like to express gratitude to the researchers and scholars whose work has contributed to the critical evaluation of viral isolation methodologies presented in this paper. Special thanks go to Dr. Stefan Lanka (website - https://wissenschafftplus.de/) and Jamie Andrews (website - https://controlstudies.substack.com/) for their extensive control experiments, which have demonstrated the limitations of CPE as a reliable indicator of viral presence. Their findings have provided essential insights into the role of experimental conditions in inducing CPE, independent of viral infection.
Additionally, sincere appreciation is extended to Aldhissla (https://substack.com/@aldhissla) for his invaluable contribution in identifying and compiling historical studies where control experiments showed that CPE can be obtained in uninoculated cultures. Their work has been instrumental in highlighting the inconsistencies and methodological flaws within virology.
Finally, the author acknowledges all researchers and scholars who have contributed to the ongoing discussion and critical reassessment of virological methodologies. Their dedication to scientific integrity and rigorous investigation continues to challenge conventional paradigms and encourage a more robust approach to virus isolation and disease research.
References
North, M. (2025). EVALUATION OF CYTOPATHIC EFFECTS IN UNINFECTED CELL CULTURES UNDER VARYING FETAL BOVINE SERUM AND ANTIBIOTIC CONCENTRATIONS. Scribd. Retrieved from https://www.scribd.com/document/820822547/Evaluation-of-Cytopathic-Effects-in-Uninfected-Cell-Cultures-Under-Varying-Fetal-Bovine-Serum-and-Antibiotic-Concentrations
Ivanovsky, D. (1892). ON THE MOSAIC DISEASE OF THE TOBACCO PLANT. St. Petersburg Academy of Sciences.
Beijerinck, M. W. (1898). CONCERNING A CONTAGIUM VIVUM FLUIDUM AS CAUSE OF THE SPOT DISEASE OF TOBACCO LEAVES. Proceedings of the Royal Netherlands Academy of Arts and Sciences.
Rivers, T. M. (1937). VIRUSES AND KOCH’S POSTULATES. Journal of Bacteriology, 33(1), 1–12.
Dolskiy, A. A., Grishchenko, I. V., & Yudkin, D. V. (2020). CELL CULTURES FOR VIROLOGY: USABILITY, ADVANTAGES, AND PROSPECTS. International Journal of Molecular Sciences, 21(21), 7978. Retrieved January 5, 2025, from https://www.mdpi.com/1422-0067/21/21/7978
Huang, C.-G., Lee, K.-M., Hsiao, M.-J., Yang, S.-L., Huang, P.-N., Gong, Y.-N., ... & Shih, S.-R. (2020). CULTURE-BASED VIRUS ISOLATION TO EVALUATE POTENTIAL INFECTIVITY OF CLINICAL SPECIMENS TESTED FOR COVID-19. Journal of Clinical Microbiology, 58(8). Retrieved January 5, 2025, from https://jcm.asm.org/content/58/8/e00961-20
Virology Research Services. (2024). WHAT IS CELL CULTURE, AND HOW HAS IT EVOLVED? Virology Research Services. Retrieved January 5, 2025, from https://www.virologyresearchservices.com/cell-culture-evolution
Fisheries. (2025). SCREENING METHOD FOR VIRAL ISOLATION. Fisheries. Retrieved January 3, 2025, from https://units.fisheries.org/fhs/wp-content/uploads/sites/30/2017/08/S2-4.5-Screening-Method-for-Viral-Isolation-2007_2014.pdf
Practical Medical Virology. (2025). VIRUS ISOLATION AND CULTIVATION. Practical Medical Virology. Retrieved January 3, 2025, from https://fac.ksu.edu.sa/sites/default/files/450_mbio_4_virus_isolation_and_cultivation2.pdf
Biology LibreTexts. (2025). ISOLATION, CULTURE, AND IDENTIFICATION OF VIRUSES. Biology LibreTexts. Retrieved January 3, 2025, from https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_%28OpenStax%29/06%3A_Acellular_Pathogens/6.03%3A_Isolation_Culture_and_Identification_of_Viruses
DeSales. (2025). ISOLATION, CULTURE, AND IDENTIFICATION OF VIRUSES. DeSales. Retrieved January 3, 2025, from https://oer.pressbooks.pub/microbilogy/chapter/isolation-culture-and-identification-of-viruses/
WOAH. (2025). VIRUS ISOLATION. WOAH. Retrieved January 3, 2025, from https://rr-asia.woah.org/app/uploads/2020/11/8-virus-isolation-li-shuo.pdf
Lanka, S. (2022). CONTROL EXPERIMENT PHASE 1 - SEVERAL LABORATORIESCONFIRM THE REFUTATION OF VIROLOGY BY THE CYTOPATHICEFFECT. scribd. Retrieved from: https://www.scribd.com/document/822261755/Control-Experiment-Phase-1-Dr-Stefan-Lanka
North, M. (2025). REEVALUATING VIRAL TRANSMISSION: A CRITICAL EXAMINATION OF VIROLOGICAL METHODS AND ASSUMPTIONS. Scribd. Retrieved from https://www.scribd.com/document/824238572/Reevaluating-Viral-Transmission-A-Critical-Examination-of-Virological-Methods-and-Assumptions

















Too bad you can not submit this paper to any scientific website or organization and ever get it published. Try getting it into an American school of medicine as part of their curriculum. Good luck! Must be very frustrating. Best wishes on breaking thru the Germ Theory wall of resistance.
Good job!