Reassessing the Foundations of Virology: A Critical Look at Historical Methods
Virus Isolation - The Three Dominant Methods Over the Past Century
1 - History of Virology
The field of virology has played a pivotal role in our understanding of ‘infectious’ diseases and developing of vaccines and treatments. However, as with any scientific discipline, it is essential to critically evaluate the methods and assumptions that underpin its progress. Over the past century, virology has relied on a variety of techniques for virus isolation, propagation, and study, ranging from early methods like in vivo serial passaging and embryonated egg propagation to more modern approaches such as cell culture and molecular biology (refer to below flow diagram).
While these methods have contributed significantly to scientific and medical ‘breakthroughs’, they are not without their limitations. Early techniques, in particular, often involved significant variability and a lack of standardization, raising questions about the reproducibility and reliability of the results they produced. For example, in vivo serial passaging—the repeated transfer of viruses between live hosts—introduced numerous uncontrolled variables, making it difficult to draw definitive conclusions about viral behavior. Similarly, the use of embryonated eggs for viral propagation, for vaccine production is inherent to a lot of variables that can influence results.
As virology has evolved, newer methods have sought to address these limitations by offering more controlled and reproducible approaches. However, many of these modern techniques are built on the foundational knowledge gained from older methods, which themselves were subject to significant variability. This raises important questions about the validity of the data on which newer techniques are based and whether the field has fully addressed the methodological inconsistencies of its past.
This article mainly examines the historical methods used in virology, with a focus on their scientific rigor and reproducibility and gives a brief overview of modern methods. The cell culture isolation method will however not be addressed again as it has been discussed at length in my previous papers (refer to my paper here).
By critically evaluating these techniques, we aim to highlight the limitations of virological research. Through this analysis, we hope to contribute to a broader discussion about the future of virology and the need for more robust and reliable methods in the study of so called viruses.
2 - In Vivo Serial Passaging
In vivo serial passaging is a technique in which a virus is repeatedly transferred between live hosts, allowing it to evolve and adapt over multiple generations. This method was claimed to increase virulence, adapt viruses to new hosts, or attenuate them for vaccine development. Throughout the late 19th and 20th centuries, this technique was instrumental in understanding viral evolution, developing animal models for human diseases, and creating some of the first ‘live-attenuated’ vaccines.
One of the most well-known studies using in vivo serial passaging is Louis Pasteur's work on the rabies vaccine in the late 1800s. Pasteur used serial passaging to attenuate the rabies virus by passing it through rabbits. He claimed that this process weakened the virus, making it less virulent while still capable of inducing an immune response in humans. His work led to the development of the first rabies vaccine and laid the foundation for modern virology and vaccine development.
2.1 - Step-by-Step Procedure for In Vivo Serial Passaging
The method used during Pasteur’s study is as follows:
Selection of Initial Pathogen: Pasteur started with the rabies virus, which he obtained from infected animals.
Inoculation of Host: He introduced the virus into the nervous tissue of rabbits. This was done by trephination, a process where a small hole is drilled into the skull of the rabbit to place the infected nervous tissue beneath the dura mater (the outer membrane covering the brain).
Incubation Period: The virus was allowed to replicate and adapt within the rabbit for a specific period. Pasteur observed the incubation period, which initially was around 15 days.
Harvesting the Pathogen: After the incubation period, Pasteur collected the infected nervous tissue from the rabbit. This involved the following steps:
Euthanizing the Rabbit: The rabbit, after being infected with the rabies virus and showing symptoms, was euthanized to collect the necessary tissue.
Dissection: Pasteur performed a dissection to access the spinal cord and brain, where it is claimed that the rabies virus was concentrated. This required precise surgical techniques to avoid contamination and ensure the integrity of the tissue.
Extraction of Nervous Tissue: The spinal cord was carefully removed from the rabbit. Pasteur focused on the spinal cord because it contained high concentrations of the virus, which was essential for his experiments.
Desiccation: The extracted spinal cord was then dried (desiccated) for varying periods. Pasteur used a series of progressively less desiccated spinal cords to create a gradient of virus attenuation. This process involved drying the tissue in a sterile environment to reduce its virulence while preserving its ability to induce an immune response
Transfer to New Host: The harvested tissue was then used to infect a new rabbit. This process was repeated multiple times, with each cycle referred to as a "passage."
Repetition of Steps: Pasteur continued this process for 90 passages. Through repeated passaging, he claimed to have shortened the incubation period of the virus to about seven days, creating what he called a "fixed" virus.
Observation and Analysis: Throughout the process, Pasteur monitored the health and behavior of the rabbits, as well as the properties of the virus. He noted changes in virulence and the ability of the virus to induce an immune response.
Final Analysis: After sufficient passages, Pasteur used the attenuated (weakened) virus to develop a vaccine. He tested the vaccine on dogs and eventually on humans, leading to the first rabies vaccination.
2.2 - Key Aspects of Standardization Attempts
Even though there were attempts to standardize in vivo serial passaging methods in virology during the early 20th century, they were often informal and based on widely accepted laboratory practices rather than strict regulatory guidelines. Standardization efforts primarily emerged from a need to ensure reproducibility, improve attenuation of viruses for vaccines, and maintain consistent virulence in experimental models.
Host Selection & Passage Conditions
Researchers often used specific animal models (e.g., rabbits, mice, monkeys) depending on the virus being studied.
Laboratories working with the same virus tended to use similar passage numbers and intervals, but these were not always formally regulated.
Notable Early Attempts at Consistency
Louis Pasteur (late 19th century) set a precedent with controlled passage of the rabies virus in rabbits, establishing a consistent approach that others later followed.
The Rockefeller Institute (early 1900s) played a key role in refining serial passage methods for viruses like yellow fever and poliovirus, influencing standardization through widely published methodologies.
Theiler’s yellow fever vaccine development (1930s) involved serial passage in mouse and chick embryos, demonstrating how controlled passaging could lead to predictable attenuation.
Guidelines & Institutional Influence
By the 1920s–30s, major research centers (Rockefeller, Pasteur Institute, Lister Institute) often shared protocols in published papers, effectively creating informal standards.
Some journals required detailed methodological descriptions, reinforcing the use of common protocols for serial passaging.
The 1950s and Beyond: Shift to Cell Cultures
After Enders, Weller, and Robbins (1954) grew poliovirus in cell cultures, in vivo passaging became less critical.
Formal standardization efforts shifted toward cell culture methods, overseen by organizations like the WHO and NIH.
While no single global standard existed for in vivo serial passaging, repeated use of similar methodologies by leading virologists effectively created widely accepted ‘best practices’.
2.3 - Discussion on In Vivo Serial Passaging
While in vivo serial passaging played a significant role in the early development of virology and vaccine production, it is important to critically evaluate the scientific rigor of this method. The technique involves repeatedly transferring a virus between live hosts (for up to 100 times), which introduces a wide range of variables that can influence the outcome. These variables include differences in bodily makeup between hosts, and environmental conditions during experimentation. Such factors make it nearly impossible to achieve consistent and reproducible results, which are fundamental to the scientific method.
The reliance on in vivo serial passaging raises questions about the validity of conclusions drawn from these experiments. For instance, changes in viral behavior observed during passaging—such as increased virulence or attenuation—could be attributed to host-specific adaptations rather than inherent properties of the virus itself to name but one obvious variable. This lack of control over experimental variables makes it difficult to establish clear causal relationships, which are essential for robust scientific inquiry.
Moreover, the historical use of in vivo serial passaging highlights a broader issue in virology: the dependence on indirect methods to infer the existence and behavior of viruses. For example, the induction of symptoms in animals through artificial inoculation is still being interpreted as evidence of viral transmission, even though alternative explanations—such as host stress responses or contamination— is not rigorously ruled out (refer to my paper here). Similarly, the reliance on cell cultures to produce cytopathic effects (CPE) to identify viruses has been criticized for its lack of specificity (refer to my paper here).
While in vivo serial passaging was a product of its time and contributed to discoveries in the field, such as the development of the rabies vaccine, its limitations underscore the need for more controlled and reproducible methods in virology. Modern techniques, such as cell culture and molecular biology, have largely replaced in vivo methods, but the historical reliance on these approaches raises important questions about the foundational assumptions of the field. As virology continues to evolve, it is crucial to address these methodological inconsistencies to ensure the validity and reliability of future research.
3 - Embryonated Eggs
The most famous and influential study where embryonated eggs were used for viral multiplication is the 1931, Ernest Goodpasture, Alice Miles Woodruff & Carl Budding titled: Cultivation of the Virus of Vaccinia.
3.1 - Step-by-Step Procedure for Viral Passage in Embryonated Eggs
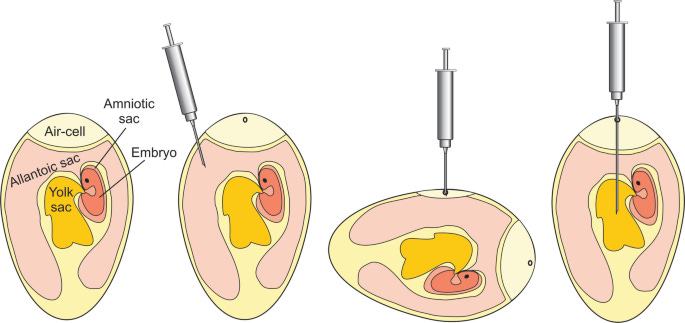
Selection of Embryonated Eggs - Use fertilized chicken eggs, typically 9–12 days old (optimal for viral growth). Eggs should be clean, disinfected, and incubated under controlled conditions (~37°C with humidity).
Candling the Egg (Determining Injection Site) - A bright light (candler) is used to locate:
The air sac (for respiratory viruses).
The allantoic cavity (for influenza virus).
The amniotic cavity (for mumps, measles, and some other viruses).
The chorioallantoic membrane (CAM) (for poxviruses).
Surface Sterilization - Wipe the shell with 70% ethanol or iodine to reduce contamination.
Inoculation of the Virus - A small drill or needle creates a hole in the eggshell at the chosen site. A fine syringe (usually 0.1–0.2 mL per egg) is used to inject the virus into the correct cavity. Common Injection Sites for Different Viruses:
Allantoic Cavity for Influenza, Newcastle disease virus
Amniotic Cavity for Influenza (for vaccine strains), Mumps virus
Chorioallantoic Membrane (CAM) for Smallpox, Vaccinia, Herpes, Poxviruses
Yolk Sac for Arboviruses (e.g., Yellow fever, Dengue), Rickettsia
Incubation - Eggs are incubated at 37°C for 24–72 hours, depending on the virus.
Embryonic death within 24 hours indicates a highly virulent strain.
Harvesting the Virus - Eggs are chilled at 4°C for several hours to reduce embryo movement and minimize contamination. The eggshell is cracked, and the infected fluid (allantoic/amniotic fluid) is extracted. Harvesting Based on Site of Infection:
Allantoic Cavity to Extract allantoic fluid
Amniotic Cavity to Extract amniotic fluid
Chorioallantoic Membrane (CAM) to Scrape off viral lesions
Yolk Sac to Extract yolk fluid
Serial Passage (Repeat the Process) - The harvested virus is diluted if needed and injected into new embryonated eggs. This process is repeated for multiple passages (e.g., 10–100 times) to:
Increase virulence (for research).
Adapt the virus to a new host.
Weaken (attenuate) the virus for vaccine development.
3.2 - Key Aspects of Standardization Attempts
There were efforts to standardize embryonated egg passaging methods in virology during the early 20th century, particularly as the technique became widely used for viral cultivation and vaccine development. These standardization attempts emerged informally through best practices in leading research institutions and were later formalized in the 1930s and 1940s.
Early Development and Informal Standardization (1910s–1920s)
Use in Avian Pathogens - The method was first used for growing fowlpox and other avian viruses, with researchers using embryonated chicken eggs as a natural medium. Techniques varied by laboratory, but early researchers often passed the virus in a way that maintained virulence or allowed attenuation.
Introduction to Mammalian Viruses
In the 1920s, scientists experimented with using eggs for viruses beyond avian pathogens, but results were inconsistent.
Formalization in the 1930s: Goodpasture & Influenza Research
1931 – Ernest Goodpasture & Alice Woodruff (Vanderbilt University) developed a reproducible method for growing fowlpox virus in the chorioallantoic membrane. This breakthrough set the stage for systematic passaging in eggs.
1936 – Wilson Smith, Christopher Andrewes, and Patrick Laidlaw successfully cultivated human influenza virus in embryonated eggs, which led to a push for standardization in viral research.
Standardization Aspects Introduced:
Egg Incubation Time: Researchers began specifying the ideal age of embryos (often 9–12 days old for influenza).
Specific Injection Sites: Different viral strains were found to grow best in certain egg compartments (allantoic cavity, amniotic cavity, chorioallantoic membrane, yolk sac).
Passage Conditions: Defined passage intervals, incubation temperatures, and sampling times to ensure reproducibility.
1940s: Standardization in Vaccine Production
With the development of the influenza vaccine (1940s, Thomas Francis Jr. & Jonas Salk), embryonated egg methods became institutionalized for large-scale production.
The World Health Organization (WHO) and U.S. National Institutes of Health (NIH) began recommending specific protocols for passaging viruses in eggs.
Evolution:
By the 1950s, cell culture methods (e.g., Enders’ poliovirus work) started replacing egg-based systems for some viruses, but embryonated eggs remained the gold standard for influenza and other select viruses.
Modern vaccine production still relies on many of these early standardization efforts, ensuring batch-to-batch consistency.
3.3 - Discussion on Embryonated Egg Passaging Methods
While the method of embryonated eggs has been claimed to be a practical solution for large-scale vaccine production, it is important to critically examine its scientific limitations and the assumptions underlying its use.
One of the primary concerns with embryonated egg passaging is the inherent variability introduced by the biological system itself. Factors such as the age of the embryo, the specific compartment of the egg used for inoculation (e.g., allantoic cavity, amniotic cavity, or chorioallantoic membrane), and the inherent biological variability among eggs can all influence claimed viral growth and adaptation. These variables make it difficult to achieve consistent and reproducible results, which are essential for rigorous scientific experimentation.
The continued reliance on this method highlights a broader issue in the field: the persistence of techniques that, does not meet the rigorous standards required for modern scientific research. For a better understanding of this procedure refer to the below video presentation.
4 - Potential Factors That Could Influence Results
My paper titled: Reevaluating Viral Transmission: A Critical Examination of Virological Methods and Assumptions (refer to Section 2 of this paper), list at least 12 different factors that could influence results of a study that attempts to prove transmission of a viral agent. These factors include the following:
Transmission Assessment Protocols:
Nasal and Oropharyngeal Swabs
Blood Sampling
Handling and Restraint of Animals
Environmental Monitoring
Invasive Monitoring Techniques
Behavioral Monitoring
Inoculation Methodological Critiques:
Intracerebral Inoculation
Intranasal and Intratracheal Inoculation
Intravenous Inoculation
Intramuscular and Subcutaneous Inoculation
Oral and Enteral Inoculation
Dermal and Transdermal Inoculation
The above variables can also be applicable for in vivo serial passaging as well as serial passaging by means of embryonated eggs. In addition to these factors one should also consider the complexity of a biological system and the multitude of factors that can influence the results of a study that claim symptoms noticed days to weeks after inoculation is due to an agent never before directly observed. It would appear that instead of finding definitive or direct proof for the existence of viruses, the field of virology had a combined vision that an agent must be the cause of their observed results and made a collaborate effort in story telling to convince the world of its existence.
5 - What The Future Holds for Virology
Virology, as a field, has undergone significant transformations over the past century, evolving from early methods like in vivo serial passaging and embryonated egg propagation to more advanced techniques such as molecular virology and synthetic biology. However, the field continues to face challenges related to the reproducibility and scientific rigor of its methods. As virology moves forward, it must address these historical inconsistencies to validate newer approaches and ensure their reliability.
In recent decades, the rise of molecular virology and recombinant DNA technology has reduced the reliance on traditional cell cultures and animal models. Techniques such as reverse genetics systems now claim to allow scientists to synthesize and study viruses at the molecular level without the need for live cell cultures.
The 2010s saw further advancements with the introduction of organoid systems and computational models. Organoids, which are miniature, simplified versions of organs grown in vitro, claim to provided a more physiologically relevant platform for studying viral infections. Meanwhile, artificial intelligence (AI) and computational models have begun to simulate viral replication and host interactions, claiming to offer new insights without the need for live cells or animal models.
The COVID-19 pandemic accelerated the adoption of alternative technologies, particularly in vaccine development. mRNA vaccine technology, exemplified by the Pfizer-BioNTech and Moderna vaccines, claim to bypass the need for traditional virus propagation in cell cultures or eggs. Instead, it is claimed that these vaccines use synthetic mRNA to instruct cells to produce viral proteins, eliciting an immune response. Similarly, CRISPR gene-editing technology is claimed to enable precise modifications to viral genomes, further reducing the reliance on traditional culturing methods.
While these advancements represent significant progress, they are not without their challenges. Many of these new methods are built on the foundational knowledge gained from older techniques, such as in vivo serial passaging, embryonated egg propagation and cell cultures. The inconsistencies and limitations of these older methods raise questions about the validity of the data on which newer techniques are based. For example, if the existence or behavior of a virus was inferred using methods with significant variability, how can we be certain that the molecular or synthetic approaches derived from these findings are accurate?
Moreover, researchers like Jamie Andrews have demonstrated that some of the same inconsistencies observed in older methods may also be present in newer techniques. In his article "Is Virology a Red Herring?", Andrews highlights potential issues with the reproducibility and specificity of modern virological methods, suggesting that the field is still grappling with foundational challenges. This raises important questions about whether newer methods are truly overcoming the limitations of their predecessors or simply inheriting their flaws.
As virology continues to evolve, it must confront these methodological challenges head-on. The field will need to demonstrate that newer techniques can overcome the limitations of older methods and provide more reliable and reproducible results. This will require rigorous validation, transparency in methodology, and a willingness to critically re-examine foundational assumptions.
5 - References and further reading:
Primary Historical Studies on Virology Methods:
Enders, J. F., Weller, T. H., & Robbins, F. C. (1954). Cultivation of poliomyelitis viruses in tissue culture. Science, 109(2830), 85-87.
Goodpasture, E. W., Woodruff, A. M., & Buddingh, G. J. (1931). Cultivation of the virus of fowlpox in the chorioallantoic membrane of the developing chick embryo. American Journal of Pathology, 7(3), 209-222.
Pasteur, L. (1885). Method for the attenuation of the rabies virus and its application to vaccination. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 101, 765-772.
Smith, W., Andrews, C. H., & Laidlaw, P. P. (1933). A virus obtained from influenza patients. The Lancet, 222(5732), 66-68.
Theiler, M., & Smith, H. H. (1937). The use of yellow fever virus modified by in vitro cultivation for human immunization. The Journal of Experimental Medicine, 65(6), 787-800.
Articles and Online Sources Referenced or Reviewed in the Document:
Andrews, J. (Jan 2025). Is virology a red herring? Substack. Retrieved from https://controlstudies.substack.com/p/is-virology-a-red-herring
Mathew North. (Feb 2025a). The non-specificity of cytopathic effects in virology. Substack. Retrieved from https://mathewnorth.substack.com/p/the-non-specificity-of-cytopathic
Mathew North. (Feb 2025b). Reevaluating viral transmission: A critical assessment. Substack. Retrieved from https://mathewnorth.substack.com/p/reevaluating-viral-transmission-a
Aldhissla (Feb 2025). Pasteur's rabies deception. Substack. Retrieved from https://aldhissla.substack.com/p/pasteurs-rabies-deception
Agent131711 (Jan 2025). How to Make a FLU VIRUS + Free Recipe Card Included, Substack. Retrieved from https://open.substack.com/pub/chemtrails/p/1940s-flu-vaccine-how-to-make-a-virus
Spackman, E., & Killian, M. L. (2020). Avian Influenza Virus Isolation, Propagation, and Titration in Embryonated Chicken Eggs. In E. Spackman (Ed.), Animal Influenza Virus (pp. 149–164). Methods in Molecular Biology (Vol. 2123). Springer. https://doi.org/10.1007/978-1-0716-0346-8_12








Virology is pure pseudoscience. Viruses are pure fiction. Transmission of disease by ‘pathogenic microbe’ is pure fiction.
I offer £3000 every day for any evidence any infectious biological pathogen has ever existed.
Needless to say - 3 years and still none offered.
Hi Matthew,
Thanks for sharing!
Whenever there is a "history of virology" or "great moments in virology science" there is an amazing lacuna.
One fundamental theory is that the dead/inactive "viruses" enter cells and then hijack cell functions (of a variety of cells) for their own replication.
This is an extraordinary theory, which, if virology was a science, would require extraordinary proof.
However, there is no science behind this theory. If there were, c. 1950s there would have been specific experiments that, non-circular, replicable, demonstrated that this occurred.
Never happened.
Simply accepted as "science" by osmosis and peer accolades.
Conclusion, virology is not really a science.
Note that this in a sense precedes the other missing links, such as isolation, infection, transmission. In a sense it is more fundamental, since it is a core definition of viruses that they have this amazing capability.
Your thoughts?
Thanks!
Steven Avery
Dutchess County, NY USA
https://linktr.ee/stevenavery