The Circular Reasoning in Virology: Common Logical Fallacies in Virus Detection
By Matthew North - 3 April 2025
Note: This paper demonstrates that there is no supplementary virus detection method independent of cell culture. Therefore, if it can be shown, through control studies like those conducted by Dr. Stefan Lanka and Jamie Andrews, that the cell culture isolation method is flawed, it would undermine the foundations of virology as a whole.
This study can be downloaded in pdf format by following this link
The Circular Reasoning in Virology: Common Logical Fallacies in Virus Detection
Matthew North1
1USA, matthewnorth@finmail.com
Date: 3 April 2025
Keywords: Viral isolation, Cytopathic effects (CPE), Cell Culture, Supplementary Viral Detection Methods
Abstract. The detection of viruses has long relied on the cell culture isolation method, which is considered the gold standard in virology. However, this method and its supplementary techniques have been criticized for their inherent circular reasoning. This paper aims to expose the circular reasoning present in current virus detection methods. By analyzing the development and application of supplementary methods we demonstrate that these techniques are fundamentally dependent on the cell culture isolation method. This dependency creates a logical fallacy, as the supplementary methods are used to confirm results derived from the very method they are supposed to validate. The paper highlights the implications of this circular reasoning for the field of virology and calls for a critical reassessment of current virus detection practices. This paper underscores the need for more robust and independent methods to identify viral presence and urges the scientific community to rethink and refine virus detection methodologies to avoid logical inconsistencies.
1 - Introduction
The detection and identification of viruses have traditionally relied on various methods, with the cell culture isolation method being the cornerstone of virological research. This method involves isolating viruses by cultivating them in controlled cell cultures, observing cytopathic effects (CPE), and subsequently purifying and identifying the virus. Despite its widespread use, the cell culture isolation method has faced criticism for its inconsistencies and inaccuracies [1, 2].
Historically, older isolation methods such as in vivo serial passaging and the use of embryonated eggs were employed for virus detection. However, these methods have been largely abandoned due to their unscientific nature and lack of reliability. The focus has since shifted to the cell culture isolation method, which, despite its flaws, remains a fundamental technique in virology.
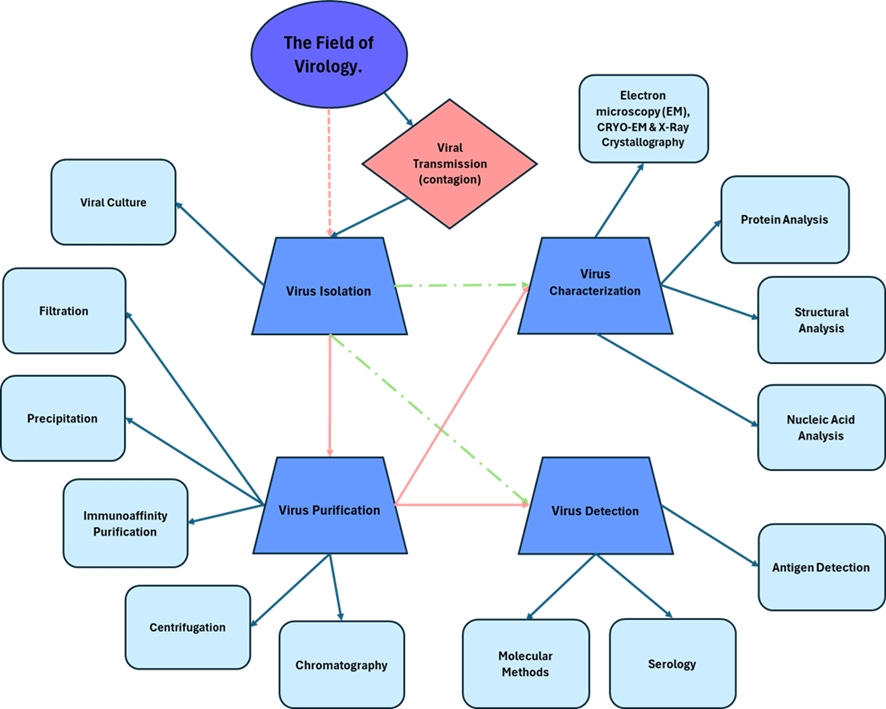
A significant issue with supplementary virus detection methods is the role of the cell culture isolation method that was utilized in the development and refining of these methods. These supplementary methods become a circular argument in the process of virus identification. The supplementary techniques that depend on the cell culture isolation technique are summarized in the graphic below.
Figure 1: Overview of key methods in virology for virus identification that has its origin in the cell culture isolation method.
This paper aims to expose the circular reasoning present in current virus detection methods. By analyzing the development and application of supplementary methods we demonstrate that these techniques are fundamentally dependent on the cell culture isolation method. This dependency creates a logical fallacy, as the supplementary methods are used to confirm results derived from the very method they are supposed to validate.
The implications of this circular reasoning for the field of virology are significant. It raises questions about the reliability and validity of current virus detection practices and highlights the need for more robust and independent methods. Through a detailed review of literature the common misconceptions among experts become evident. This paper underscores the importance of re-evaluating and refining virus detection methodologies to avoid logical inconsistencies and improve the accuracy of virological research.
2 - Literature Review
2.1 - Historical Methods of Virus Detection
Historically, virus detection methods included in vivo serial passaging and the use of embryonated eggs. These techniques, although once prevalent, have been criticized for their lack of scientific rigor and reliability. In vivo serial passaging involves the repeated infection of live animals to propagate the virus, while embryonated eggs are used to cultivate viruses within the developing embryo. Both methods have been largely abandoned due to their unscientific nature and the ethical concerns associated with their use [3].
2.2 - Cell Culture Isolation Method
The cell culture isolation method has become the cornerstone of modern virology. This technique involves isolating viruses by cultivating them in controlled cell cultures and observing cytopathic effects (CPE). Despite its widespread use, the cell culture isolation method is claimed to have its limitations which include, its susceptibility to contamination, low sensitivity for low viral load and due to some viruses not growing in culture. Supplementary virus detection methods are therefore suggested to confirm the presence of a virus.
2.3 - Supplementary Methods and Circular Reasoning
All supplementary methods used to detect viruses have been developed based on the cell culture isolation method (refer to Figure 1). These supplementary methods create a dependency on the cell culture isolation method, leading to circular reasoning and the methods are used to confirm results derived from the very method they are supposed to validate. This is known as a methodological circularity, and it raises questions about their reliability and validity.
This literature review provides a comprehensive overview of the historical and current methods of virus detection, highlighting the need for more reliable and independent techniques to accurately identify viral presence.
3 – Methodology
The cell culture isolation method has been discussed in length in previous papers [1, 2]. This paper will however have its focus on exposing circular reasoning in supplementary virus detection methods due to their inherent dependence on the cell culture isolation method. An overview of the supplementary methods analyzed is summarized in Figure 1.
The data for this study is collected from a comprehensive review of existing literature, published peer-reviewed studies and historical methods of virus detection. The analysis focuses on identifying instances of circular reasoning in the development and application of supplementary virus detection methods.
Literature Review: A thorough review of historical and current virus detection methods is conducted to provide context and background for the study.
Case Studies: Specific examples of circular reasoning are examined in detail to illustrate the dependency on the cell culture isolation method.
To aid in the understanding of the central role of the cell culture isolation method in virology, visual representations are included. These visuals illustrate the dependency of supplementary methods on the cell culture isolation method and highlight the circular reasoning present in current virus detection practices.
This study employs a critical analysis of the cell culture isolation method and its supplementary techniques to expose circular reasoning in virus detection. By highlighting the logical fallacies and dependencies inherent in current practices, the study aims to advocate for the development of more robust and independent virus detection methods.
4 - Results
The analysis of the cell culture isolation method and its supplementary techniques reveals several key findings that highlight the circular reasoning present in current virus detection practices. In this section supplementary virus detection methods are assessed to confirm its dependence on the cell culture isolation method.
4.1 - Nucleic Acid-Based Detection Methods
Modern nucleic acid-based techniques, such as Polymerase Chain Reaction (PCR), Reverse Transcription Quantitative PCR (RT-qPCR), Digital PCR (dPCR), Next-Generation Sequencing (NGS), RNA sequencing, and viral metagenomics, all stem from the need to establish viral reference genomes. These reference genomes are derived from virus samples first isolated and purified using cell cultures. This reliance creates a circular validation problem, as PCR and sequencing techniques confirm viral presence using primers and reference genomes originally established from cell culture studies (refer to Figure 2). Even CRISPR-based detection methods like SHERLOCK and DETECTR utilize guide RNAs designed from cell culture-derived viral genomes [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15].
Figure 2: The circular argument of utilizing nucleic acid-based detection methods to confirm viral presence.
4.2 - Protein-Based Detection Methods
Protein-based detection techniques, including proteomic analysis, immunogold labeling, immunohistochemistry (IHC), enzyme-linked immunosorbent assay (ELISA), and mass spectrometry-based viral protein identification, depend on virus-infected cell cultures for obtaining and characterizing viral proteins. These methods confirm viral presence based on protein markers originally identified through studies on cultured viruses, reinforcing the circular nature of their validation [16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Refer to Figure 3 which shows the circular reasoning in utilizing nucleic acid-based detection methods to confirm viral presence.
Figure 3: The circular argument of utilizing protein-based detection methods to confirm viral presence.
4.3 - Visualization Techniques
Electron microscopy (EM), immunofluorescence assays (IFA), and immunohistochemistry methods require high concentrations of viral particles for accurate imaging. These concentrations are typically achieved through virus propagation in cell cultures. The viral structures observed under EM and related techniques were initially characterized from cultured virus samples, making these methods dependent on the cell culture isolation process [23, 24, 25, 26, 27, 28, 29]. Refer to Figure 4 which shows the circular reasoning in utilizing visualization methods to confirm viral presence.
Figure 4: The circular argument of utilizing visualization techniques to confirm viral presence.
4.4 - Antibody-Based Detection Methods
Lateral flow immunoassays (LFIA), hemagglutination (HA), hemagglutination inhibition (HI), viral neutralization assays, and plaque assays all require viral proteins or whole viruses obtained from cultured samples to develop antibodies or test reagents. These methods confirm viral presence by detecting proteins that were originally characterized in cell culture experiments, contributing to methodological circularity [28, 30, 31, 32, 33, 34, 35, 36]. Refer to Figure 5 which shows the circular reasoning in utilizing antibody-based detection methods to confirm viral presence.
Figure 5: The circular argument of utilizing antibody-based detection methods to confirm viral presence.
4.5 - Functional and Biochemical Assays
Viral replication assays, cytotoxicity assays, viral pseudotype neutralization, and viral interference assays rely on the behavior of viruses in cultured cells to assess infectivity, replication, and interaction with host cells. These methods fundamentally depend on cell culture experiments, making them inherently circular in their validation approach [28, 29, 30, 31, 32]. Refer to Figure 6 which shows the circular reasoning in utilizing functional and biochemical assays to confirm viral presence.
Figure 6: The circular argument of utilizing functional and biochemical assays to confirm viral presence.
4.6 - Advanced and Emerging Detection Technologies
Biosensors, microarrays, surface plasmon resonance (SPR), and nanotechnology-based detection techniques use viral markers such as proteins and nucleic acids that were first identified using cell culture-grown viruses. Even cutting-edge techniques like single-molecule detection, viral genome integration assays, and CRISPR-Cas-based detection ultimately rely on reference materials that originated from cell culture studies [37, 38, 39, 40, 41, 42].
4.15 - Implications for Virology
Figure 7 illustrates the central role of the cell culture isolation method in virology. This visual highlight the dependency of supplementary methods on the cell culture isolation method and the circular reasoning present in current virus detection practices.
The findings of this study have significant implications for the field of virology. The circular reasoning present in current virus detection methods raises questions about the reliability and validity of these practices. The study underscores the need for more robust and independent methods to accurately identify viral presence. The common misconceptions among experts who encourage the use of these supplementary methods further highlight the importance of re-evaluating and refining virus detection methodologies to avoid logical inconsistencies and improve the accuracy of virological research.
Figure 7: Overview of key methods in virology for virus identification that has its origin in the cell culture isolation method.
5 - Discussion
The analysis of the cell culture isolation method and its supplementary techniques reveals significant issues of circular reasoning in current virus detection practices. This section discusses the implications of these findings for the field of virology and highlights the need for more robust and independent methods.
5.1 - Implications of Circular Reasoning
The reliance on the cell culture isolation method as the foundation for developing supplementary virus detection techniques, creates a logical fallacy. These supplementary methods are used to confirm results derived from the cell culture isolation method, leading to circular reasoning. This dependency undermines the reliability and validity of the results, as the methods are not truly independent.
The implications of this circular reasoning are profound. It raises questions about the accuracy of current virus detection practices and the conclusions drawn from them. If the foundational method is flawed or inconsistent, then the supplementary methods built upon it are also likely to be unreliable. This has significant consequences for virological research, diagnostics, and public health responses.
5.2 - Expert Misconceptions
The insistence that viral presence cannot be concluded solely based on cell culture experiments and that it must be supplemented with other methods, highlights a common misconception among experts in the field. This critique underscores the need for a critical reassessment of current virus detection practices. The reliance on supplementary methods that are fundamentally dependent on the cell culture isolation method demonstrates a lack of understanding of the logical fallacies involved.
The insistence on using supplementary methods without recognizing their inherent dependency on the cell culture isolation method suggests a need for better education and awareness among virologists and researchers. It is essential to critically evaluate the methods used in virus detection and ensure that they are truly independent and reliable.
5.3 - Need for Robust and Independent Methods
The findings of this study highlight the urgent need for the development of more robust and independent virus detection methods. Current practices, which rely heavily on the cell culture isolation method and its supplementary techniques, are fraught with logical inconsistencies. To improve the reliability and accuracy of virus detection, it is crucial to develop methods that do not depend on the cell culture isolation method.
Future research should focus on exploring alternative techniques that can independently verify viral presence without relying on the results of cell culture experiments. This may involve the development of new technologies or the refinement of existing methods to eliminate the dependency on the cell culture isolation method.
The analysis of the cell culture isolation method and its supplementary techniques exposes significant issues of circular reasoning in current virus detection practices. The implications of these findings are far-reaching, raising questions about the reliability and validity of virological research and diagnostics. To improve the accuracy and reliability of virus detection, it is essential to develop more robust and independent methods that avoid logical inconsistencies. This study serves as a call to action for the scientific community to critically reassess current practices and strive for more reliable and accurate virus detection techniques.
6 - Summary
This study critically examines the cell culture isolation method and its supplementary techniques, revealing significant issues of circular reasoning in current virus detection practices. The analysis demonstrates that supplementary methods are fundamentally dependent on the cell culture isolation method. This dependency creates a logical fallacy, as these methods are used to confirm results derived from the very method they are supposed to validate, leading to circular reasoning.
Every supplementary virus detection method has been developed based on prior cell culture isolation studies. Whether it’s PCR, RNA sequencing, proteomic analysis, immunogold labeling, or even direct mass spectrometry approaches, each method relies on reference materials, genetic sequences, or antigen characterizations that were originally obtained through cultured viruses.
Even so-called "culture-independent" methods, like NGS of clinical samples or CRISPR-based virus detection, were only possible because the foundational viral genomes, protein structures, and antigenic properties were first established using cell culture techniques.
Thus, there is no modern viral detection method that can be utilized independent of the cell culture isolation method. Every current detection approach is inherently linked to virological work that used cell cultures to define and validate viral markers.
The implications of this circular reasoning are profound, raising questions about the reliability and validity of current virus detection practices. The common misconceptions among experts who recommend the use of these supplementary methods to confirm the results of the cell culture isolation method underscores the need for a critical reassessment of current methodologies. The reliance on supplementary methods that are inherently dependent on the cell culture isolation method highlights a lack of understanding of the logical inconsistencies involved.
To improve the accuracy and reliability of virus detection, it is essential to develop more robust and independent methods. Future research should focus on exploring alternative techniques that can independently verify viral presence without relying on the results of cell culture experiments. This may involve the development of new technologies or the refinement of existing methods to eliminate the dependency on the cell culture isolation method.
7 – Conclusion
In conclusion, this study exposes significant issues of circular reasoning in current virus detection practices and calls for a critical reassessment of these methodologies. By highlighting the logical fallacies and dependencies inherent in current practices, the study advocates for the development of more reliable and independent virus detection methods, if at all possible.
8 - Acknowledgements
I would like to extend my deepest gratitude to the individuals and groups whose work has significantly contributed to the critical examination and reevaluation of virological methodologies and assumptions presented in this paper.
Finally, I extend my appreciation to all the researchers, scientists, and supporters who have contributed to this critical evaluation. Your commitment to scientific integrity and the pursuit of truth continues to inspire and drive meaningful progress.
References
North, M. (2025). THE NON-SPECIFICITY OF CYTOPATHIC EFFECTS: IMPLICATIONS FOR VIROLOGICAL RESEARCH AND PUBLIC HEALTH. Scribd. Retrieved from https://www.scribd.com/document/827764224/The-Non-Specificity-of-Cytopathic-Effects-Implications-for-Virological-Research-and-Public-Health-RV0
North, M. (2025). EVALUATION OF CYTOPATHIC EFFECTS IN UNINFECTED CELL CULTURES UNDER VARYING FETAL BOVINE SERUM AND ANTIBIOTIC CONCENTRATIONS. Scribd. Retrieved from https://www.scribd.com/document/820822547/Evaluation-of-Cytopathic-Effects-in-Uninfected-Cell-Cultures-Under-Varying-Fetal-Bovine-Serum-and-Antibiotic-Concentrations
North, M. (2025, March 9). REASSESSING THE FOUNDATIONS OF VIROLOGY. Substack. https://mathewnorth.substack.com/p/reassessing-the-foundations-of-virology
Mullis, K. B., & Faloona, F. A. (1987). SPECIFIC SYNTHESIS OF DNA IN VITRO VIA A POLYMERASE-CATALYZED CHAIN REACTION. Methods in Enzymology, 155, 335-350.
Saiki, R. K., et al. (1988). PRIMER-DIRECTED ENZYMATIC AMPLIFICATION OF DNA WITH A THERMOSTABLE DNA POLYMERASE. Science, 239(4839), 487-491.
Bustin, S. A., et al. (2009). THE MIQE GUIDELINES: MINIMUM INFORMATION FOR PUBLICATION OF QUANTITATIVE REAL-TIME PCR EXPERIMENTS. Clinical Chemistry, 55(4), 611-622.
Sanger, F., Nicklen, S., & Coulson, A. R. (1977). DNA SEQUENCING WITH CHAIN-TERMINATING INHIBITORS. Proceedings of the National Academy of Sciences, 74(12), 5463-5467.
Margulies, M., et al. (2005). GENOME SEQUENCING IN MICROFABRICATED HIGH-DENSITY PICOLITRE REACTORS. Nature, 437(7057), 376-380.
Metzker, M. L. (2010). SEQUENCING TECHNOLOGIES - THE NEXT GENERATION. Nature Reviews Genetics, 11(1), 31-46.
Wang, D., et al. (2003). VIRAL DISCOVERY AND SEQUENCE RECOVERY USING DNA MICROARRAYS. PLoS Biology, 1(2), e2.
Shendure, J., & Ji, H. (2008). NEXT-GENERATION DNA SEQUENCING. Nature Biotechnology, 26(10), 1135-1145.
Kitamura, N., et al (1981). PRIMARY STRUCTURE, GENE ORGANIZATION AND POLYPEPTIDE EXPRESSION OF POLIOVIRUS RNA. Nature, 291(5816), 547-553.
Metzker, M. L. (2010). SEQUENCING TECHNOLOGIES — THE NEXT GENERATION. Nature Reviews Genetics, 11(1), 31-46.
Gootenberg, J. S., et al (2017). NUCLEIC ACID DETECTION WITH CRISPR-CAS13A/C2C2. Science, 356(6336), 438-442.
Chen, J. S., et al (2018). CRISPR-CAS12A TARGET BINDING UNLEASHES INDISCRIMINATE SINGLE-STRANDED DNASE ACTIVITY. Science, 360(6387), 436-439.
Aebersold, R., & Mann, M. (2003). MASS SPECTROMETRY-BASED PROTEOMICS. Nature, 422, 198-207.
Washburn, M. P., Wolters, D., & Yates, J. R. (2001). LARGE-SCALE ANALYSIS OF THE YEAST PROTEOME BY MULTIDIMENSIONAL PROTEIN IDENTIFICATION TECHNOLOGY. Nat Biotech, 19, 242-247.
Cox, J., & Mann, M. (2008). MAXQUANT ENABLES HIGH PEPTIDE IDENTIFICATION RATES, INDIVIDUALIZED P.P.B.-RANGE MASS ACCURACIES AND PROTEOME-WIDE PROTEIN QUANTIFICATION. Nature Biotechnology, 26(12), 1367-1372.
Gstaiger, M., & Aebersold, R. (2009). APPLYING MASS SPECTROMETRY-BASED PROTEOMICS TO GENETICS, GENOMICS AND NETWORK BIOLOGY. Nature Reviews Genetics, 10(9), 617-627.
Roth, J., Bendayan, M., & Orci, L. (1978). ULTRASTRUCTURAL LOCALIZATION OF INTRACELLULAR ANTIGENS BY THE USE OF PROTEIN A-GOLD COMPLEX. J Histochem Cytochem, 26(12):1074-81
Slot, J. W., & Geuze, H. J. (1985). A NEW METHOD OF PREPARING GOLD PROBES FOR MULTIPLE-LABELING CYTOCHEMISTRY. European Journal of Cell Biology, 38(1), 87-93.
Griffiths, G. (1993). FINE STRUCTURE IMMUNOCYTOCHEMISTRY. Springer-Verlag.
Coons, A. H., Creech, H. J., & Jones, R. N. (1941). IMMUNOLOGICAL PROPERTIES OF AN ANTIBODY CONTAINING A FLUORESCENT GROUP. Proceedings of the Society for Experimental Biology and Medicine, 47(2), 200-202.
Sternberger, L. A., Hardy, P. H., Cuculis, J. J., & Meyer, H. G. (1970). THE UNLABELED ANTIBODY ENZYME METHOD OF IMMUNOHISTOCHEMISTRY: PREPARATION AND PROPERTIES OF SOLUBLE ANTIGEN-ANTIBODY COMPLEX (HORSERADISH PEROXIDASE-ANTIHORSERADISH PEROXIDASE) AND ITS USE IN IDENTIFICATION OF SPIROCHETES. Journal of Histochemistry and Cytochemistry, 18(5), 315-333.
Hsu, S. M., Raine, L., & Fanger, H. (1981). USE OF AVIDIN-BIOTIN-PEROXIDASE COMPLEX (ABC) IN IMMUNOPEROXIDASE TECHNIQUES: A COMPARISON BETWEEN ABC AND UNLABELED ANTIBODY (PAP) PROCEDURES. Journal of Histochemistry and Cytochemistry, 29(4), 577-580.
Morgan, C., Rose, H. M., & Moore, D. H. (1956). STRUCTURE AND DEVELOPMENT OF VIRUSES AS OBSERVED IN THE ELECTRON MICROSCOPE. Journal of Experimental Medicine, 104(2), 171-182.
Horne, R. W., & Wildy, P. (1961). SYMMETRY IN VIRUS ARCHITECTURE. Virology, Volume 15, Iss 3, Pages 348-373,
Hsiung G.D., Melnick J.L. PLAQUE FORMATION WITH POLIOMYELITIS, COXSACKIE, AND ORPHAN (ECHO) VIRUSES IN BOTTLE CULTURES OF MONKEY EPITHELIAL CELLS. Virology. 1955 Dec;1(5):533-5.
Dales S. (1962); AN ELECTRON MICROSCOPE STUDY OF THE EARLY ASSOCIATION BETWEEN TWO MAMMALIAN VIRUSES AND THEIR HOSTS. J Cell Biol, 13 (2): 303–322.
Dulbecco, R. (1952). PRODUCTION OF PLAQUES IN MONOLAYER TISSUE CULTURES BY SINGLE PARTICLES OF AN ANIMAL VIRUS. Proceedings of the National Academy of Sciences, 38(8), 747-752.
Dulbecco, R., Vogt, M. (1954). PLAQUE FORMATION AND ISOLATION OF PURE LINES WITH POLIOMYELITIS VIRUSES. Journal of Experimental Medicine, 99(2), 167-182.
Fenner, F., & Woodroofe, G. M. (1960). The reactivation of poxviruses: II. The range of reactivating viruses. Virology, 11(1), 185-201.
Fazekas de St. Groth, S., & Webster, R. G. (1966). DISQUISITIONS ON ORIGINAL ANTIGENIC SIN: I. EVIDENCE IN MAN. Journal of Experimental Medicine, 124(3), 331-345.
Webster, R. G., & Laver, W. G. (1967). PREPARATION AND PROPERTIES OF ANTIBODY DIRECTED SPECIFICALLY AGAINST THE NEURAMINIDASE OF INFLUENZA VIRUS. Journal of Immunology, 99(1), 49-55.
Hirst, G. K. (1942). THE QUANTITATIVE DETERMINATION OF INFLUENZA VIRUS AND ANTIBODIES BY MEANS OF RED CELL AGGLUTINATION. Journal of Experimental Medicine, 75(1), 49-64.
Posthuma-Trumpie, G. A., Korf, J., & van Amerongen, A. (2009). LATERAL FLOW (IMMUNO)ASSAY: ITS STRENGTHS, WEAKNESSES, OPPORTUNITIES AND THREATS. A LITERATURE SURVEY. Analytical and Bioanalytical Chemistry, 393(2), 569-582.
Chiu, C. Y., et al (2008). UTILITY OF DNA MICROARRAYS FOR DETECTION OF VIRUSES IN ACUTE RESPIRATORY TRACT INFECTIONS IN CHILDREN. The Journal of Pediatrics, 153(1), 76-83.
Kistler, A., et al (2007). PAN-VIRAL SCREENING OF RESPIRATORY TRACT INFECTIONS IN ADULTS WITH AND WITHOUT ASTHMA REVEALS UNEXPECTED HUMAN CORONAVIRUS AND HUMAN RHINOVIRUS DIVERSITY. Journal of Infectious Diseases, 196(6), 817-825.
Palacios, G., et al (2007). PANMICROBIAL OLIGONUCLEOTIDE ARRAY FOR DIAGNOSIS OF INFECTIOUS DISEASES. Emerging Infectious Diseases, 13(1), 73-81.
Saylan, Y., Erdem, Ö., Ünal, S., & Denizli, A. (2019). AN ALTERNATIVE MEDICAL DIAGNOSIS METHOD: BIOSENSORS FOR VIRUS DETECTION. Biosensors, 9(2), 65.
Qiu, G., et al (2020). DUAL-FUNCTIONAL PLASMONIC PHOTOTHERMAL BIOSENSORS FOR HIGHLY ACCURATE SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 DETECTION. ACS Nano, 14(5), 5268-5277.
Layqah, L. A., & Eissa, S. (2019). AN ELECTROCHEMICAL IMMUNOSENSOR FOR THE CORONA VIRUS ASSOCIATED WITH THE MIDDLE EAST RESPIRATORY SYNDROME USING AN ARRAY OF GOLD NANOPARTICLE-MODIFIED CARBON ELECTRODES. Microchimica Acta, 186(4), 224.









🚨Introducing the Revolutionary 'Viral Isolation™ Method' 🚨
Because Who Needs the Scientific Method When You Have Consensus™?
Are you a virologist looking to isolate a virus but don’t want to deal with pesky scientific principles? Do you long for a world where assumptions are facts, controls are optional, and results are whatever you need them to be? Well, look no further! With Viral Isolation™, you, too, can bypass every fundamental step of the scientific method and still get published!
🎉 Special Features – Now with ZERO Adherence to the Scientific Method! 🎉
🔬 Step 1: Skip Direct Observation!
Why waste time finding a virus in nature when you can just assume it exists? Don’t worry—no one in ‘virology’ has ever observed a virus as a distinct, independent entity directly from a sick person’s fluids. If they haven’t done it in over a century, why start now?
💡 Bonus: Instead of isolating a virus, just declare symptoms = virus. Got a cough? Must be a virus! Fever? Definitely a virus! Ate bad sushi? Yep - virus!!
📏 Step 2: The Hypothesis-Free Hypothesis™!
Real science requires an Independent Variable (IV) (the thing being tested), but who has time for that? Since we never actually isolate a virus, we’ll just assume it’s there. Science is hard—so let’s just skip the part where we identify our IV!
🧐 Scientific Method Violation:
✅ No purified virus? No problem! Just declare the existence of one and move on.
✅ Symptoms are vague? Who cares? A fever must mean viral infection!
✅ What about controls? HAHAHA, good one.
🧪 Step 3: Experimental Design... Or Lack Thereof!
Time to “prove” viral replication! But instead of isolating and testing a virus, let’s throw patient samples into a blender of monkey kidney cells, toxic antibiotics, fetal bovine serum, and a battery of chemicals! What could go wrong?
🧬 Pro-Tip: When the monkey kidney cells start dying from all the poison you added, just call it a Cytopathic Effect (CPE) and claim the virus did it! (Ignore the fact that control experiments show the same results without a virus—just pretend those don’t exist!)
📸 Step 4: The Electron Microscope Magic Show!
Now, let’s get some ‘proof.’ Instead of isolating and purifying a virus, just snap a picture of the cellular debris from our CPE Toxicity Soup™ and say, "See? That blob right there—totally a virus."
🔍 Fun Fact: If someone asks why you didn’t purify and separate the virus, just roll your eyes and mutter, “You don’t understand virology.” That’ll shut them up!
📊 Step 5: Data Manipulation & Narrative Control!
Science should be about unbiased analysis... but where’s the fun in that? With Viral Isolation™, you can:
✅ Assemble ‘viral genomes’ on a computer, even if no complete ‘genome’ was found! (Computers never make mistakes.)
✅ Use PCR to ‘detect’ tiny ‘genetic fragments,’ from a ‘virus’ never proven to exist.
✅ Ignore all contradictory evidence, and call anyone who questions your methods a science denier™!
🧠 Step 6: Declare Success No Matter What!
In real science, if an experiment fails, the hypothesis should be rejected. But with Viral Isolation™, failure is never an option!
🚫 Found no actual virus? Publish anyway!
🚫 Control experiments contradict your results? Ignore them!
🚫 No reproducibility? Silence the skeptics!
🌟 SPECIAL BONUS! – Support the Digital Biosecurity State! 🌟
By believing in this groundbreaking Non-Scientific Methodology™, YOU, too, can:
💰 Enable the Biosecurity State™ – The more viruses we ‘find,’ the more lockdowns, mandates, and surveillance we can justify!
📉 Help Crush Small Businesses! – Nothing says ‘progress’ like eliminating those pesky local shops and centralizing power!
🛑 Contribute to The Great Reset™! – Because why have freedom when you can have digital IDs, carbon quotas, and endless health passports?
🚀 ORDER NOW! 🚀
For a limited time, get your Viral Isolation™ Kit for the low price of your critical thinking skills!
(Side effects include cognitive dissonance, FOIA-induced panic attacks, and an uncontrollable urge to shout, “Trust the Science!” at anyone who asks for evidence.)
💥 Viral Isolation™ – Where Assumptions Become Facts!💥
Round and Round they go. Even if the indirect methods were legit, they're not, they would still need to demonstrate contagion in a real world environment. They would need to expose well people to these invisible particles via natural methods and observe the same symptoms they claim the particles cause.